
- #Polymath software multiple reactions manual#
- #Polymath software multiple reactions software#
- #Polymath software multiple reactions Pc#
- #Polymath software multiple reactions license#
- #Polymath software multiple reactions plus#
It can also execute as a DOSapplication under Windows 3.1, Windows 95, and Windows NT.
#Polymath software multiple reactions Pc#
POLYMATH workswith PC and MS DOS 3.0 and above.
#Polymath software multiple reactions plus#
The minimum desirable application memory is450 Kb plus extended memory for large applications. Most graphics boards areautomatically supported. POLYMATH runs on the IBM Personal Computer and most com-patibles. The authors of POLYMATH shall not be liable for any damages,including special, indirect, incidental, or consequential damages, withrespect to any claim arising out of or in connection with the use of thesoftware even if users have not been or are hereafter advised of thepossibility of such damages.
#Polymath software multiple reactions software#
This software is provided AS IS, WITHOUT REPRESENTATION AS TOITS FITNESS FOR AND PURPOSE, AND WITHOUT WARRANTY OFANY KIND, EITHEREXPRESS OR IMPLIED, including with limitationthe implied warranties of merchantability and fitness for a particularpurpose. Internetdistribution is not allowed under any circumstances. Permission to otherwise copy, distribute, modifyor otherwise create derivative works of this software is prohibited.
#Polymath software multiple reactions license#
This individual-use license is for POLYMATH Version 4.02 and applies tothe owner of the textbook.
#Polymath software multiple reactions manual#
One copy of the manual may be reproduced in hard copy only fornoncommercial educational use of the textbook owner. Only one copy of thissoftware is to be in use on only one computer or computer terminal at anyone time. This license is fornoncommercial and educational uses exclusively. The authors of POLYMATH agree to license the POLYMATH materialscontained within this disk and the accompanying manual.pdf file to theowner of the Prentice Hall textbook Elements of Chemical ReactionEngineering, Third Edition, by H. This manual may be reproduced for educational purposes by licensedusers. IBM and PC-DOS are trademark of International Business MachinesMS-DOS and Windows are trademarks of Microsoft CorporationĬopyright 1998 by M. This will include updates on this version and availabil-ity of future versions. Users are encouraged to obtain the latest general informa-tion on POLYMATH and its use from the above Internetsite. Scott Fogler and published by Prentice Hall THIS MANUAL AND SOFTWARE ARE TO ACCOMPANYElements of Chemical Reaction Engineering - 3rd Editionīy H. Use the algorithm to solve the remainder of the problem.- SIMULTANEOUS LINEAR EQUATIONS- POLYNOMIAL, MULTIPLE LINEAR AND NONLINEAR REGRESSIONįor IBM and Compatible Personal Computers Three Forms of the Mole Balance Applied to Semibatch Reactors: 1. The reactant that starts in the reactor is always the limiting reactant. Semibatch reactors can be very effective in maximizing selectivity in liquid phase Polymath will combine for you- Thanks Polymath.you rock! The membrane is permeable to Product C, but it isįor membrane reactors, we cannot use conversion. To all other species, is placed around the reacting mixture.Įxample: The following reaction is to be carried out isothermally in a membrane ToĪccomplish this, a membrane that is permeable to that reaction product, but is impermeable
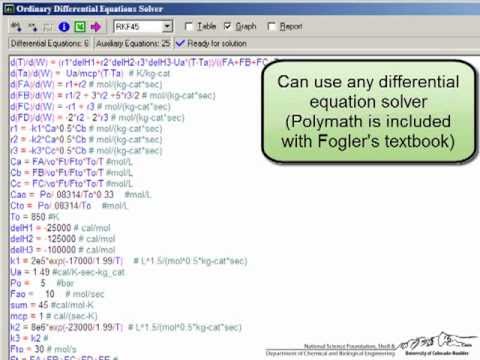
You can remove one of the reaction products and drive the reaction to the right. These higher conversions are the result of Le Chatelier's Principle Membrane reactors can be used to achieve conversions greater than the originalĮquilibrium value. University of Washington Transport Effects in Microreactors site If the flow is laminar, we must use the techniques discussed in chapter 13. What Four Things are Wrong With this solution?įor isothermal microreactors, we use the same equations as a PFR as long as the flow is not laminar. To answer these questions we need to see how a varies with these parameters.

(See Critical Thinking in Preface page xx. "If we double the particle size, decrease the porosity by a factor of 3, andĭouble the pipe size, what will happen to D P and X?"

We want to learn how the various parameters (particle diameter, porosity, etc.) affect In CD-ROM chapter 12, we will learn that effectiveness factor decreases as the particle size increasesĪnalysis - Critical Thinking and Creative Thinking Therefore, there are more entry ways into the catalyst particle. For a given catalyst weight, there is a greater external surgace area for smaller particles than larger particles. The larger the particle, the more time it takes the reactant to get in and out of the catalyst particle. The specific reaction rate decreases as the particle size increases, therefore so deos the conversion. Increasing the particle diameter descreases the pressure drop and increases the rate and conversion. "What Four Things are Wrong with this Solution?"


 0 kommentar(er)
0 kommentar(er)
